
Seasonal and Long-Term Variability in Well Water NO3-N
S.B.Phillips, W.R. Raun, and G.V. Johnson
Oklahoma State University
Abstract
Nitrate contamination of groundwater is a growing concern worldwide. Considering that analytical methods have changed over the past forty years, data collected using 1950's techniques has been incorrectly compared to data collected today. Without applying random and bias errors associated with each independent method, these reported changes in NO3-N concentrations are scientifically invalid. This study was initiated to determine the errors associated with analytical procedure, seasonal sampling, and storage method for well water NO3-N analysis. Nitrate-N concentrations for fifty water wells in Grant, Garfield, and Kingfisher counties, obtained during the period 1950-1972, were acquired through cooperation with The United States Geological Survey and The Oklahoma Water Resources Board. Well water samples were taken at different dates from these same locations in 1993 and 1994. For each sampling date, four samples were taken from each well whereby two were frozen immediately (as per EPA protocol), and two were stored at ambient temperature for five to ten days as was common in 1950. Water samples were then analyzed using phenoldisulfonic acid (1950) and automated cadmium reduction(1993) procedures. Mean NO3-N values from analysis using cadmium reduction were significantly higher than values obtained using the phenoldisulfonic acid procedure, reflecting the bias associated with making direct numerical comparisons between procedures. Comparisons made between 1950 and 1993 well water analysis (employing the same phenoldisulfonic acid procedure) indicate that few significant increases have taken place over the past forty years. No relationship between well water NO3-N and depth to aquifer was found which indirectly suggests that NO3-N leaching was not significant for this population of 50 wells.
Introduction
Nitrate-N contamination of groundwater is a growing concern worldwide. When nitrate-nitrogen(NO3-N) is ingested by infants, nitrate can be reduced to nitrite. Nitrite-N can occupy sites on hemoglobin that would normally carry oxygen. This reduced oxygen carrying capacity of the blood is what leads to methemoglobinemia, or "blue baby" syndrome which can be fatal to infants under six months of age. This condition is, however, treatable and fatalities are rare. In the last thirty years, only one death in the United States was linked to Nitrate poisoning caused from drinking well water (Fedkiw, 1991). The maximum level for NO3-N in drinking water is 10 mg/kg(as set by the Environmental Protection Agency). Results from a national survey (National Pesticide Survey, 1990) noted that only a small percentage (2.4%) of rural water wells exceed this level.
The issue that groundwater NO3-N concentrations have increased over time is not disputed. Nitrate-N occurs naturally in the soil (Johnson, 1993). Despite this fact, agricultural chemicals, particularly N fertilizers, have received the bulk of the blame for the increase. A computer based literature search performed by L.W. Canter found 34 references where groundwater NO3-N was associated with fertilizer use (Canter, 1987). In order for fertilizers to be directly responsible for groundwater contamination, NO3-N would have to be leached out of the soil profile. The occurrence of this is preceded by other processes resulting in much higher levels of N removal (Mills et al., 1974; Sharpe et al., 1988; Hooker et al., 1980; Aulakh et al., 1984). DeWalle and Schaff, 1980, Hallberg, 1983, and Olsen ,1974, among many others, have reported agriculture related increases in groundwater NO3-N concentrations over time periods of up to thirty years. Considering that analytical methods have changed since the 1950's, direct numerical comparison between data obtained thirty years ago and data obtained today is erroneous. Schepers et al., 1991, Walters and Malzer, 1990, and Webster et al., 1986, have also evaluated the impacts of N fertilizers without addressing the analytical errors associated with these estimates. Without adequately assessing the random and bias errors associated with an independent estimate, researchers are at risk of making scientifically invalid conclusions about changes in NO3-N concentrations.
The objective of this research was to determine the errors associated with analytical procedure, seasonal sampling, and storage method for well water NO3-N analysis. A thorough understanding of the errors associated with estimates of NO3-N in groundwater will assist researchers in identifying where significant increases have taken place.
Materials and Methods
Fifty water wells in Garfield, Grant, and Kingfisher counties (Figure 1) were selected for comprehensive sampling. These wells were selected on the basis that NO3-N data collected during the time period 1953-1972 was available (Bingham and Bergman, 1980; Dover, 1953; U.S.G.S., 1993). These counties also have substantial agricultural activity associated with continuous wheat production and N fertilization. Locations and physical characteristics for each well site are found in Table 1. Tax records obtained from the three counties were used to determine current ownership of the property on which each well was located. The owners were contacted and informed about the experiment. Permission was obtained from the well owners to begin quarterly well water sampling beginning in the fall of 1993.
In order to obtain a representative groundwater sample, it is desirable to take the sample directly from the aquifer. However, 39 of the 50 wells contain in-place semi-permanent mounted pumps which limit the options available for groundwater sampling. These wells were pumped for 5-10 minutes prior to sampling so that water in the well reasonably represented that of the aquifer. Of the 11 other wells, 7 were collected via windmills and 4 were collected using a teflon bailer. All samples were taken using proper sampling protocol (Barcelona et al., 1987; Davis et al., 1993; Scalf et al., 1981). For each sampling date, four samples were taken from each well whereby two were frozen immediately (AOAC, 1984) by being placed in a cooler containing dry ice and two were stored at ambient temperatures for five to ten days as was common in 1950.
Frozen and non-frozen samples were analyzed using two methods. One method was phenoldisulfonic acid (Bremner, 1965; Chapman and Pratt, 1961; Snell, 1949), a procedure commonly used in 1950 when the original NO3-N data was collected. The other method, automated cadmium reduction (Henriksen and Selmer-Olsen, 1970; Jackson et al., 1975), was performed using the Lachat-Quickchem automated flow injection system (1993). Statistical analysis of data was performed using procedures outlined by the SAS institute (SAS, 1988). Geographic Information Systems was used to produce surface and contour maps showing relationships among wells and analytical methods.
Results and Discussion
Significant differences (.0001 probability) in well water NO3-N were found between season of sampling and method of analysis. The mean NO3-N value for samples taken in each of the four seasons ranged from 6.97 ± 5.8 in the fall to 9.59 ± 8.65 in the summer. This agrees with work done by Gilliam et al., 1974, Hallberg et al., 1983, and McDonald and Splinter, 1982, all of whom found NO3-N levels for a specific well to vary among seasons. This reflects a bias error associated with seasonal sampling. The difference between frozen and non-frozen samples was statistically insignificant (.8415 probability). The largest difference in means was found when comparisons were made between analytical methods. Mean well water NO3-N concentrations found by using the phenoldisulfonic acid procedure were 3.2 mg/kg lower than those found using cadmium reduction (9.4 ± 7.5 vs 6.2 ± 4.7). Possible explanations for this difference are that a loss of NO3-N occurred when using the phenoldisulfonic acid procedure, or that the accuracy of phenoldisulfonic acid is poor in terms of NO3-N detection. In either case, an error exists. Comparing population means generated by all possible combinations of the variables (season, storage, and method) resulted in overlapping standard deviations which seriously restricted whether or not conclusive changes had taken place when comparing data obtained using independent methods (Figure 2). Comparisons made between 1950 and 1993 well water analysis (employing the same phenoldisulfonic acid procedure) indicated that 57 % of all wells sampled have shown either a significant decrease or no significant change at all over the past forty years (Table 2).
No relationship was found between the average depth to aquifer and well water NO3-N in either 1953, 1993 or the difference (1993-1953, Figure 3). Because fertilizer use was not common before 1950, increased fertilizer use from 1950-present should have resulted in significant well water NO3-N increases in more shallow aquifers if in fact NO3-N leaching from surface applied fertilizers was present. If NO3-N leaching from the excessive use of N fertilizers were to have been significant over this forty year time period, depth to the aquifer (on these extremely shallow wells, <30 ft) and well water NO3-N should have been negatively correlated.
References
Aulakh, M.S., D.A. Rennie, and E.A. Paul. 1984. The influence of plant residues on denitrification rates in conventional and zero tilled soils. Soil Sci. Soc. Am. J. 48:790-794.
Bremner, J.M. 1965. Inorganic forms of nitrogen. In C.A. Black et al. (ed.) Methods of soil analysis, Part 2. Agronomy 9:1179-1237. Am. Soc. of Agron., Inc., Madison, WI.
Canter, L.W. 1987. Nitrates and Pesticides in Groundwater: An Analysis of a Computer Based Literature Search. In Deborah M. Fairchild (ed.) Groundwater Quality and Agricultural Practices. Lewis Publishers. Chelsea, MI.
Dewalle, F.B., and R.M. Schaff. 1980. Groundwater pollution by septic tank drainfields. J. Environmental Engineering Division. 106(EE3):631-648.
Dover, T.B. 1953. Chemical character of public water supplies in Oklahoma, 1953. Bulletin No. 8. Division of Water Resources, Oklahoma Planning and Resources Board. U.S. Geological Survey.
Fedkiw, John. 1991. Nitrate occurrence in U.S. waters, a reference summary of published sources from an agricultural perspective. United States Department of Agriculture, Washington DC.
Hallberg, G.R. et al. 1983. Hydrogeology, Water Quality , and Land Management in the Big Spring Basin, Clayton County, Iowa. Open file report No. 83-3. Iowa Geological Survey. Iowa City, Iowa.
Henriksen, H., and A.A. Selmer‑Olsen. 1970. Automatic methods for determining nitrate and nitrite in water and soil extracts. Analyst 95:514‑518
Hooker M.L., D.H. Sander, G.A. Peterson, and L.A. Diagger. 1980. Gaseous N losses from winter wheat. Agron. J. 72:789-792.
Jackson, W.A., C.E. Frost, and D.M. Hildreth. 1975. Versatile multi‑range analytical manifold for automated analysis of nitrate‑N. Soil Sci. Soc. Am. Proc. 39:592‑593
Johnson, G.V. 1993. Fate of Fertilizer Nitrogen in Soils. Environmental Notes. Oklahoma State University Center for Agriculture and the Environment, Stillwater, OK.
Milham, P.J., A.S. Awad, r.E. Paull, and J.H. Bull. 1970. Analysis of plants, soils and waters for nitrate by using an ion-selective electrode. Analyst. 95:751-757.
Mills, Harry A., Allen V. Barker, and Donald N. Maynard. 1974. Ammonia volatilization from soils. Agron. J. 66:355-358.
National Research Council. Committee on Nitrate Accumulation. 1972. Fertilizer and Soil Nitrogen. p. 40. In: Accumulation of Nitrate. National Academy of Sciences. Washington, D.C.
Official Methods of Analysis of the Association of Official Analytical Chemists. 1984. Association of Official Analytical Chemists, Inc., Arlington, VA.
Patterson, H.D., and B.I. Lowe. 1970. The errors of long-term experiments. J. Agric. Sci. (Cambridge) 74:53-60.
Schepers, J.S., M.G. Moravek, E.E. Alberts, and K.D. Frank. 1991. Maize production impacts on groundwater quality. J. Environ. Qual. 20:12-16.
Sharpe, R.R., L.A. Harper, J.E. Giddens, and G.W. Langdale. 1988. Nitrogen use efficiency and nitrogen budget for conservation tilled wheat. Soil Sci. Soc. Am. J. 52:1394-1398.
Snell, F.D., and C.T. Snell. 1949. Colorimteric methods of analysis, Vol. 2. 3rd ed. D. Van Nostrand Co., New York.
United States Department of Agriculture Extension Service. 1990. National Pesticide Survey; Phase One Report. United States Department of Agriculture, Washington DC.
Walters, D.T., and G.L. Malzer. 1990. Nitrogen management and nitrification inhibitor effects on nitrogen-15 urea: II. Nitrogen leaching and balance. Soil Sci. Soc. Am. J. 54:122-130.
Webster, C.P., R.K. Belford, and R.Q. Cannell. 1986. Crop uptake and leaching losses of 15N labeled fertilizer nitrogen in relation to waterlogging of clay and sandy loam soils. Plant Soil 92:89-101.
Table 1. Location, soil type, major land use and average depth to aquifer for each well sampled in 1953 and 1993, Grant, Garfield and Kingfisher Counties, OK.
|
WELL |
COUNTY |
LOCATION |
SOIL TYPE |
MAJOR LAND USE |
AVG. DEPTH TO AQUIFER |
|
|
|
|
|
|
IN FEET BELOW SURFACE |
|
1 |
KINGFISHER |
15N-07W-02 CC |
KINGFISHER SILT LOAM, 3 TO 5 % SLOPE |
CROPLAND |
10 |
|
2 |
KINGFISHER |
16N-07W-29 C |
RENFROW CLAY LOAM, 1 TO 3 % SLOPE |
CROPLAND |
9 |
|
3 |
KINGFISHER |
17N-07W-36 B |
NORGE FINE SANDY LOAM, 0 TO 1 % SLOPE |
CROPLAND |
19 |
|
4 |
KINGFISHER |
17N-06W-31 B |
PORT SILT LOAM, 0 TO 1 % SLOPE |
RANGELAND |
19 |
|
5 |
KINGFISHER |
17N-05W-31 D |
KINGFISHER SILT LOAM, 3 TO 5 % SLOPE |
CROPLAND |
35 |
|
6 |
KINGFISHER |
17N-05W-32 B |
PORT CLAY LOAM, 0 TO 1 % SLOPE |
CROPLAND |
35 |
|
7 |
KINGFISHER |
17N -05W-32 C |
PORT CLAY LOAM, 0 TO 1 % SLOPE |
CROPLAND |
35 |
|
8 |
KINGFISHER |
17N-05W-35 D |
PRATT LOAMY FINE SAND, UNDULATING |
CROPLAND |
22 |
|
9 |
KINGFISHER |
17N-05W-36 A |
PRATT LOAMY FINE SAND, UNDULATING |
CROPLAND |
27 |
|
10 |
KINGFISHER |
17N-05W-18 D |
PRATT LOAMY FINE SAND, HUMMOCKY |
CROPLAND |
25 |
|
11 |
KINGFISHER |
17N-07W-11 D |
LINCOLN LOAMY FINE SAND |
IRRIGATED PASTURELAND |
10 |
|
12 |
KINGFISHER |
17N-08W-4 C |
NORGE-SLICKSPOT COMPLEX, 1 TO 3 % SLOPE |
CROPLAND |
7 |
|
13 |
KINGFISHER |
17N-07W-06 B |
NORGE FINE SANDY LOAM, 0 TO 1 SLOPE |
CROPLAND |
5 |
|
14 |
KINGFISHER |
17N-07W-01 C |
PRATT LOAMY FINE SAND, HUMMOCKY |
RANGELAND |
7 |
|
15 |
KINGFISHER |
18N-06W-32 B |
PRATT LOAMY FINE SAND, UNDULATING |
CROPLAND |
28 |
|
16 |
KINGFISHER |
18N-07W-28 C |
PRATT LOAMY FINE SAND, UNDULATING |
CROPLAND |
11 |
|
17 |
KINGFISHER |
18N-07W-24 A |
DOUGHERTY-EUFALA LOAMY FINE SAND, HUMMOCKY |
IRRIGATED CROPLAND |
29 |
|
18 |
KINGFISHER |
18N-07W-13 AC |
CARWILE LOAMY FINE SAND |
CROPLAND |
25 |
|
19 |
KINGFISHER |
18N-07W-15 A |
SHELLABARGER FINE SANDY LOAM, 5 TO 8 % SLOPE |
RANGELAND |
26 |
|
20 |
KINGFISHER |
18N-07W-08 D |
DOUGHERTY-EUFALA LOAMY FINE SAND, UNDULATING |
CROPLAND |
26 |
|
21 |
KINGFISHER |
18N-08W-02 D |
PRATT LOAMY FINE SAND, HUMMOCKY |
CROPLAND |
18 |
|
22 |
KINGFISHER |
18N-O8W-02 C |
DOUGHERTY-EUFALA LOAMY FINE SAND, HUMMOCKY |
PASTURELAND |
18 |
|
23 |
KINGFISHER |
19N-07W-29 D |
DOUGHERTY-EUFALA LOAMY FINE SAND, UNDULATING |
CROPLAND |
33 |
|
24 |
KINGFISHER |
19N-07W-8 D |
CARWILE LOAMY FINE SAND |
CROPLAND |
6 |
|
25 |
KINGFISHER |
19N-08W-11 DA |
DOUGHERTY-EUFALA LOAMY FINE SAND, HUMMOCKY |
IRRIGATED PASTURELAND |
13 |
|
26 |
KINGFISHER |
19N-08W-11 A |
DOUGHERTY-EUFALA LOAMY FINE SAND, HUMMOCKY |
IRRIGATED PASTURELAND |
13 |
|
27 |
KINGFISHER |
15N-07W-02 C |
ALLUVIAL AND BROKEN LAND |
CROPLAND |
7 |
|
28 |
KINGFISHER |
19N-09W-23 BBB |
EUFALA FINE SAND |
CROPLAND |
11 |
|
29 |
KING FISHER |
19N-09W-10 AB |
PRATT LOAMY FINE SAND, UNDULATING |
RANGELAND |
17 |
|
30 |
KINGFISHER |
19N-08W-05 B |
DOUGHERTY-EUFALA LOAMY FINE SAND, UNDULATING |
CROPLAND |
8 |
|
31 |
GARFIELD |
20N-08W-23 CC |
SHELLABARGER FINE SANDY LOAM, 1 TO 3 % SLOPE |
CROPLAND |
20 |
|
32 |
GARFIELD |
20N-05W-28 A |
PORT CLAY LOAM |
CROPLAND |
18 |
|
33 |
GARFIELD |
20N-03-W-26 BBC |
ERODED CLAYEY LAND |
RANGELAND |
38 |
|
34 |
GARFIELD |
21N-04W-11 D |
KIRKLAND SILT LOAM, 0 TO 1 % SLOPE |
CROPLAND |
19 |
|
35 |
GARFIELD |
21N-06W-12 CCB |
PORT SILT LOAM, 0 TO 1 % SLOPE |
CROPLAND |
18 |
|
36 |
GARFIELD |
21N-07W-23 BBC |
POND CREEK SILT LOAM, 0 TO 1 % SLOPE |
CROPLAND |
18 |
|
37 |
GARFIELD |
21N-08W-20 CCC |
DRUMMOND SOILS |
RANGELAND |
11 |
|
38 |
GARFIELD |
22N-O8W-18 A |
GRANT-NASH SILT LOAM, 5 TO 8 % SLOPE |
RANGELAND |
3 |
|
39 |
GARFIELD |
22N-07W-01 D |
SHELLABARGER-CARWILE FINE SANDY LOAM, UNDULATING |
URBAN/BUILT UP LAND |
18 |
|
41 |
GARFIELD |
24N-03W-05 B |
RENFROW-VERNON COMPLEX, 3 TO 5 % SLOPE, ERODED |
CROPLAND |
10 |
|
42 |
GARFIELD |
24N-05W-03 C |
KIRKLAND-RENFROW SILT LOAM, 1 TO 3 % SLOPE |
CROPLAND |
18 |
|
43 |
GARFIELD |
24N-05W-35 A |
KIRKLAND SILT LOAM, 0 TO 1 % SLOPE |
CROPLAND |
17 |
|
44 |
GARFIELD |
23N-06W-7 D |
MENO LOAMY FINE SAND, UNDULATING |
RANGELAND |
20 |
|
45 |
GARFIELD |
23N-07W-7 C |
POND CREEK SILT LOAM, 0 TO 1 % SLOPE |
CROPLAND |
27 |
|
46 |
GARFIELD |
24N-07W-21 ADD |
GRANT SILT LOAM, 3 TO 5 % SLOPE, ERODED |
CROPLAND |
18 |
|
48 |
GRANT |
25N-05W-6 D |
YAHOLA FINE SANDY LOAM, OCCASIONALLY FLOODED |
RANGELAND |
10 |
|
49 |
GRANT |
28N-07W-24 A |
QUINLAN-WOODWARD LOAM, 3 TO 12 % SLOPE |
RANGELAND |
6 |
|
50 |
GRANT |
27N-03W-28 B |
RENFROW SILTY CLAY LOAM, 2 TO 5 % SLOPE, ERODED |
CROPLAND |
26 |
|
51 |
GRANT |
27N-03W-22 B |
McLAIN-DRUMMOND SILT LOAM, RARELY FLOODED |
RANGELAND |
30 |
|
52 |
GRANT |
27N-03W-23 A |
KIRKLAND SILT LOAM, 1 TO 3 % SLOPE |
CROPLAND |
30 |
|
|
|
|
|
|
|
Table 2. Nitrate-N concentrations for each well sampled between 1950 and 1972 and the 1993 sampling data analyzed using the phenoldisulfonic acid procedure when samples were not frozen, Grant, Garfield and Kingfisher Counties, OK.
______________________________________________________________________________
1993 1950-72
Well No. NO3-N SD NO3-N Sig.
______________________________________________________________________________
3 8.80 0.20 0.02 **
4 2.90 0.16 42.70 **
5 9.06 0.10 .11 **
6 2.52 0.16 .18 ns
7 12.20 0.06 .02 **
8 14.60 0.03 .14 **
9 8.24 0.06 6.75 ns
10 7.61 0.10 8.78 ns
11 0.45 0.13 0.00 ns
12 10.00 0.25 0.16 **
14 12.40 0.16 0.29 **
15 6.76 0.41 . ns
16 11.10 0.65 11.30 ns
17 18.20 0.76 . ns
18 11.90 0.98 5.85 **
19 10.00 0.03 36.00 **
20 7.89 0.02 3.83 **
21 3.04 0.30 0.29 *
22 6.54 0.36 . ns
23 7.10 0.30 2.70 **
24 12.00 2.21 1.31 **
25 13.40 0.10 67.50 **
26 12.50 0.92 38.30 **
29 5.38 0.13 4.50 ns
31 15.20 0.79 5.85 **
32 2.33 0.12 0.77 ns
34 5.78 0.05 1.91 **
35 6.41 0.01 2.00 **
36 3.58 0.03 1.13 ns
37 5.38 0.00 1.24 **
38 1.19 0.18 22.50 **
41 14.30 0.21 6.98 **
42 0.61 0.40 6.75 **
43 1.02 0.21 0.38 ns
44 11.40 0.36 2.25 **
45 5.17 0.17 1.76 *
46 2.65 0.03 3.38 ns
48 1.98 0.25 0.02 ns
49 1.26 0.54 1.91 ns
51 6.97 0.46 13.50 **
52 0.54 0.31 5.85 **
______________________________________________________________________________
**, * - significant decrease at 0.01 and 0.05 probability levels respectively.
**, * - significant increase at 0.01 and 0.05 probability levels respectively.
ns - not significant.
43% significant increase
20% significant decrease
37% no significant change
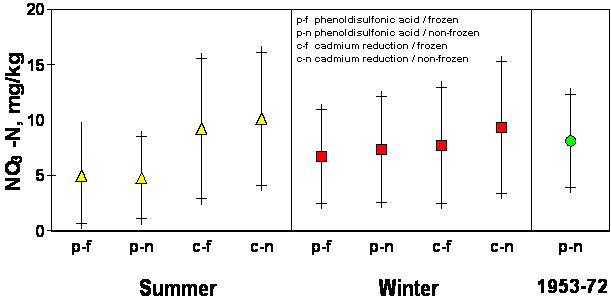
Figure 2.
Nitrate-N analysis by season, analytical procedure and storage method from well
water samples collected in Grand, Garfield and Kingfisher Counties, OK,
1953-1993.
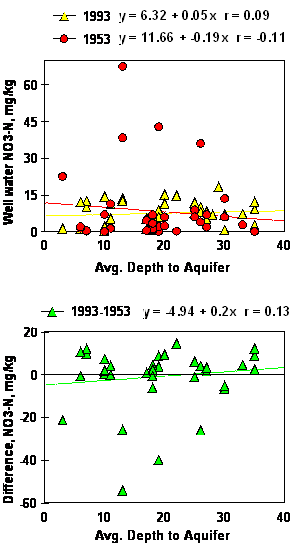
Figure 3. Simple linear regression of well water NO3-N on the average depth to aquifer, 1993, 1953 and the difference between 1993 and 1953.